Category: Gryphon
Assessing the Risks and Benefits of Conducting Research on Pathogens of Pandemic Potential: Supplemental Material–Animal Models for Coronaviruses and Influenza Viruses
Assessing the Risks and Benefits of Conducting Research on Pathogens of Pandemic Potential: Supplemental Material–Landscape of GoF and Alt-GoF Research
Assessing the Risks and Benefits of Conducting Research on Pathogens of Pandemic Potential: Supplemental Information–Notable and Selected Recent Avian Influenza Outbreaks
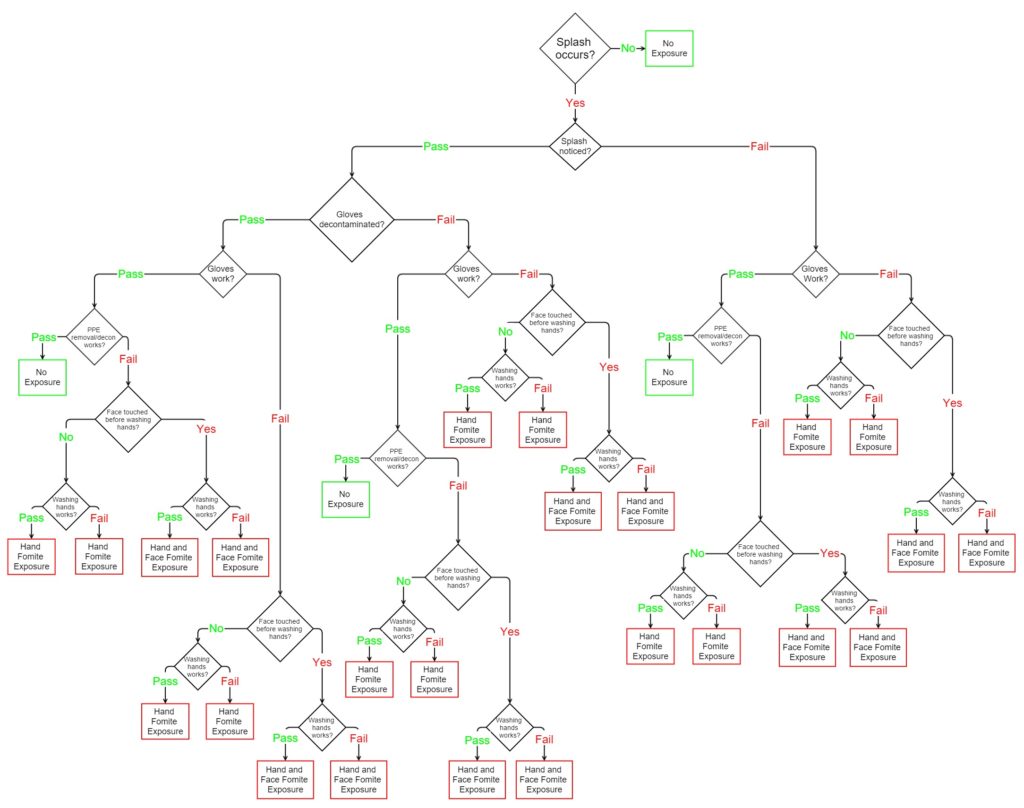
Assessing the Risks and Benefits of Conducting Research on Pathogens of Pandemic Potential: Supplemental Information–Minute Tidal Volume of Ducks
Assessing the Risks and Benefits of Conducting Research on Pathogens of Pandemic Potential: Supplemental Information–Duck Populations
Assessing the Risks and Benefits of Conducting Research on Pathogens of Pandemic Potential (2016)
In October 2014, the White House Office of Science and Technology Policy (OSTP) announced a funding pause on selected “Gain of Function” (GoF) research involving influenza viruses, SARS coronavirus, and MERS coronavirus, namely experiments that are “reasonably anticipated to confer attributes to influenza, MERS, or SARS viruses such that the virus would have enhanced pathogenicity and/or transmissibility in mammals via the respiratory route” (White House OSTP Moratorium Memo). OSTP called for a deliberative process to evaluate the risks and potential benefits of this research, which would culminate in the development and adoption of a new US Government (USG) policy governing the funding and conduct of GoF research and the cessation of the funding pause. The National Science Advisory Board for Biosecurity (NSABB) served as the official federal advisory body on GoF research issues and was responsible for developing recommendations for the appropriate level of Federal oversight of GoF research. To inform the NSABB’s deliberations on this issue, Gryphon Scientific was contracted by the NIH Office of Science Policy to conduct risk and benefit assessments (RBA) of GoF research involving the pathogens subject to the funding pause. Our assessment was divided into four components: Biosafety risk assessment; Assessment of biosecurity risks due to intentional acts against the laboratory; Assessment of biosecurity risks due to misuse of information; Benefit assessment.
Supplements:
Avian Influenza-Related Supporting Information
Supplemental Information–Duck Populations
Supplemental Information–Minute Tidal Volume of Ducks
Supplemental Information–Notable and Selected Recent Avian Influenza Outbreaks
Risk & Benefit Assessment Supplemental Material
Supplemental Material–Landscape of GoF and Alt-GoF Research
Supplemental Material–Animal Models for Coronaviruses and Influenza Viruses
Supplemental Material–State of Surveillance for Influenza Viruses and Coronaviruses
Final Selected Presentations
Risk and Benefit Analysis (RBA) of Gain of Function Research
Risk and Benefit Analysis (RBA) of Gain of Function Research: Gaps and Future Considerations
Risk and Benefit Analysis (RBA) of Gain of Function Research: Summary
Risk and Benefit Analysis (RBA) of Gain of Function Research: Progress Update
Final Selected Reports
Risk and Benefit Analysis of Gain of Function Research: Draft Final Report
Risk and Benefit Analysis of Gain of Function Research: Bibliographies
Human Epidemiological and Sociological Data
Supplemental Information–Data Supporting the Disease Course of SARS and MERS
Supplemental Information–Data Supporting the Modeling of the Disease Course of Influenza
Supplemental Information–Data Supporting Antiviral and Vaccine Efficacy
Supplemental Information–Data Supporting MERS and SARS R0
Laboratory PPE, Experiment, and Animal-Related Supporting Information
Supplemental Information–Avian Influenza Titers in Mouse and Ferret Models
Supplemental Information–Approach to Estimating Salivary Titers for CoVs
Supplemental Information–Durability Assessment of Culture Flasks
Supplemental Information–Protection Afforded by PAPRs
Other Risk-Related Supplemental Information
Supplemental Information–Calculating Earthquake Risk
Supplemental Information–Protection Against Infection with 1918 H1N1 Pandemic Strain
Other Supplemental Information
Case Studies to Inform Discussions about Defining and Evaluating GoF Studies of Concern
Interview Guides for Risk and Benefit Analysis of Gain-of-Function Research
Risk Assessment Parameters
Supplemental Information–Influenza and CoV Modes of Transmission and Environmental Stability
Supplemental Information–Global Demographics Supporting SEIR Modeling
Supplemental Information–Dose Response Parameters for Gain of Function Pathogens
Supplemental Information–Fomite Model
Supplemental Information–Detailed Information of the BARDA Interactive Flu Model
Supplemental Information–Detailed Parameters of the Branching Process Model
Supplemental Information–Detailed Descriptions of the Fault Tree Analyses
Event Trees










The Accelerating Pace of the Democratization of Biotechnology (2019)
As biotechnologies mature from activities requiring substantial educational and financial investments into those requiring far less resources, the technologies can more readily be misused to cause harm. Understanding the speed at which new biotechnologies become “democratized” is important for developing regulatory and security policies and practices that safeguard against accidental or intentional misuse without unduly hampering cutting-edge research. In this publication from Nature Biotechnology, Gryphon researchers use a novel analytical method to analyze the pace of advancement of biotechnologies. Gryphon’s analysis suggests that novel biotechnologies can become democratized – that is, accessible to many individuals with relatively low levels of technical skill and financial resources – in less than 4.5 years from their discovery and may do so in less than 3.5 years by the end of the next decade. These results suggest that ongoing review of the security risks associated with biotechnologies is needed to enable proactive development of mitigation policies and oversight systems.
Promoting Biosecurity by Professionalizing Biosecurity (2020)
Advances in life sciences research and biotechnology fields, including synthetic biology, genomics, and neuroscience, are transforming the agriculture, healthcare, energy, and other sectors that rely on use of biologically derived materials. However, some of these materials, technologies, and associated information and expertise potentially could be exploited to cause harm to humans, animals, plants, the environment, public safety, or national security. Biosecurity is a multidisciplinary effort to identify and mitigate biological risks by implementing risk- and threat-based control measures to prevent the unauthorized access, misuse, loss, theft, diversion, and intentional release of such “dual use” materials, technologies, information, and expertise.
Historically, biosecurity policies and practices have focused on risks posed by pathogens and toxins, and therefore, may not address adequately the security concerns posed by emerging life sciences research and technologies. In this Policy Forum, the authors propose the establishment of a professional biosecurity credential to support the creation of a well-trained, responsible workforce with a core set of skills necessary to secure the life sciences of the future.
Access this paper here.
