Welcome to this week’s Pandora Report! This issue highlights the evolving biological threat landscape – from agroterrorism and toxin risks to AI-enabled biotechnology – alongside efforts to reinforce preparedness, governance, and global health security.
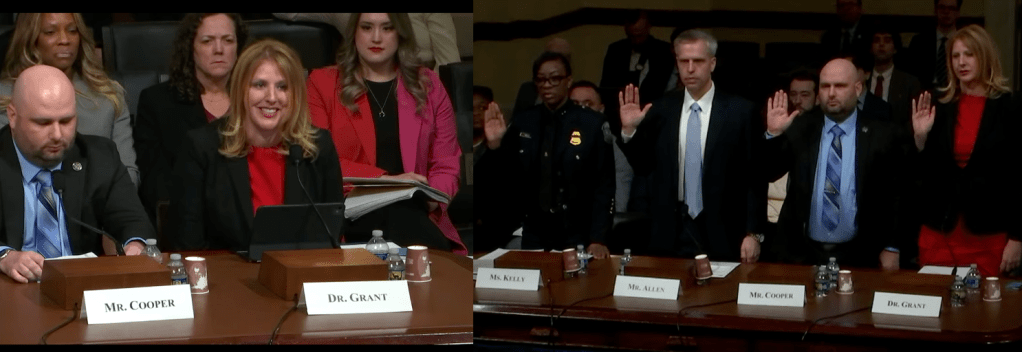
GMU Biodefense Adjunct Dr. Ashley Grant Testifies Before Congress on Agroterrorism
Dr. Ashley Grant, Senior Health Security and Biodefense Advisor, Office of Health Security, U.S. Department of Homeland Security (DHS) and adjunct professor in the George Mason University’s Biodefense Graduate Program, testified before Congress on February 11 as part of the House Committee on Homeland Security’s Subcommittee on Emergency Management and Technology hearing on, “Surveying the Threat of Agroterrorism, Part II: Assessing Federal Government Efforts.”
In her testimony, Dr. Grant outlined DHS Office of Health Security’s (OHS) coordinated approach to defend the nation’s food, agriculture, and veterinary systems against terrorism and other high-consequence threats, whether intentional or naturally occurring. She underscored the growing risks posed by agroterrorism and other biological threats, emphasizing that advances in biotechnology and artificial intelligence (AI), while beneficial, have also lowered barriers for malicious actors seeking to disrupt crops, livestock, and food production systems. “Food security is national security,” she noted.
Dr. Grant concluded by reaffirming DHS’s commitment to safeguarding the nation’s food and agriculture systems through collaboration, driving innovation, and sustained readiness of our homeland to meet the challenges ahead.
For more information on the hearing, visit here.
A Bipartisan Plan for Strengthening Defenses Against Biological Threats
The CSIS Bipartisan Alliance for Global Health Security Working Group on Biodefense has released a new report, Protecting Americans from Biological Threats, that provides a prioritized set of practical, near-term recommendations for the U.S. government to modernize biosurveillance, ensure biosafety and biosecurity, reverse the decline of the biodefense enterprise, and strengthen response and recovery. These recommendations were developed through intensive deliberations with working group members over the past four months and endorsed by a bipartisan group of over 40 expert signatories. Dr. Gregory Koblentz, Director of the Biodefense Graduate Program, was a member of the working group and one of the signatories to the final report. Sophia Hirshfield, MS Biodefense ’23, a research associate at the Center for Global Health at CSIS, was one of the report’s co-authors.
Further Reading:
- “Strengthening Preparedness Against Novel Biological Threat Agents Enabled Through Artificial Intelligence and Other Emerging Technologies,” National Academies of Sciences, Engineering, and Medicine.
- “Defensive acceleration: the strategic pivot needed for UK biological resilience,” The Centre for Long-Term Resilience.
- “Informing the European Biotech Act and Inclusions of our Recommendations,” The Centre for Long-Term Resilience
Norway’s Focus 2026 Warns of Expanding Biological Weapons Risks Amid AI Advances
Norway’s Focus 2026, an annual report published by the Norwegian Intelligence Service, is a threat and risk assessment of current security challenges. The report notes that although Russia and China are both signatories to the Biological Weapons Convention (BWC), both countries retain considerable capacity to produce biological and chemical agents due to historical expertise, dual-use research, and extensive biotechnology investments. Russia, for instance, continues using less-lethal agents such as teargas and chloropicrin in Ukraine. Meanwhile, although China signed the BWC in 1984, Chinese research institutions affiliated with the armed forces have been working to identify and test highly toxic substances for years. The report also highlights how advances in biotechnology, artificial intelligence (AI), and big data could facilitate the design of novel toxic substances and microorganisms with specific characteristics, posing evolving risks to security systems involving both humans and food production.
Further Reading:
- ‘Measuring Biological Capabilities and Risks of AI Agents: Generating and Interpreting Evidence from Agentic Evaluations,” Patricia Paskov, Jeffrey Lee, Kyle Brady, and Alyssa Worland, RAND
- “Open-source AI program can answer science questions better than humans,” Jeffrey Brainard, Science
- “mRNA Technology as a National Security Asset,” Council on Strategic Risks Blog
- “Sentinel 2025 Annual Letter,” Sentinel Bio
Bird Flu Threatens Antarctic Wildlife as Researchers Explore Vaccine Solutions
By Margeaux Malone, Pandora Report Associate Editor
Recent research has shed new light on the growing threat of highly pathogenic avian influenza (HPAI) bird flu to wildlife in some of the world’s most remote ecosystems, while also pointing toward promising new tools for conservation.
A study published in Scientific Reports, led by Erasmus MC and UC Davis, confirmed the first documented wildlife die-off from H5N1 avian influenza on the Antarctic continent. More than 50 south polar skuas, which are large, ecologically important seabirds related to gulls, were found to have died from the virus during the summers of 2023 and 2024. Scientists previously detected the virus in dead birds in Antarctica, but HPAI was not confirmed as the cause of the deaths. This research showed through a combination of virological, bacteriological, and pathological analyses that H5N1 HPAI caused multi-organ failure and death in skuas, but not in other Antarctic species found deceased around the same time. Researchers noted the virus caused severe neurological symptoms in affected birds and warned that skuas’ role as scavengers could position them to spread the disease further across the continent. The last Antarctic skua census was conducted in the 1980s and counted only around 800 breeding pairs. The loss of more than 50 birds to H5N1 could represent a significant blow to the population, though the true scale remains unclear without updated population data. Researchers warn that without increased monitoring efforts in Antarctica, the full extent of the virus’s impact on these and other vulnerable species may go undetected.
On a more encouraging note, a study published recently in Nature Communications demonstrated that vaccination against H5 avian influenza can produce a strong and lasting immune response in wild king penguins, offering a potential tool for wildlife conservation.
King penguins, which breed in dense colonies across sub-Antarctic islands and raise their chicks over an extended period, were identified as an ideal candidate species for a vaccination trial in a natural setting. In the study, thirty penguin chicks received a self-amplifying mRNA vaccine targeting the H5 hemagglutinin protein, the component of the virus responsible for entering host cells, while twenty unvaccinated chicks served as controls. Researchers monitored the birds for 250 days and found that vaccinated chicks maintained high levels of neutralizing antibodies all the way through to fledging, with no adverse effects observed. Importantly, testing confirmed that none of the birds had been naturally exposed to the virus during the trial period, meaning the immune response was driven entirely by the vaccine itself.
The research team also examined the cellular mechanisms behind the immune response, identifying significant activity in memory B cells and T cells, the components of the immune system responsible for recognizing and rapidly responding to future infections. This suggests the vaccine does not offer merely short-term protection but may prime the immune system for a durable, long-term defense. Historically, there has been little scientific data published on the ability of non-domesticated birds to sustain vaccine-induced immunity without repeated natural exposure to a virus. These findings contribute significantly to our understanding of host immunity in Antarctic avifauna and may be adaptable to other wild and domestic species globally.
Together, these developments emphasize the expanding ecological footprint of avian influenza and the growing importance of coordinated surveillance, research, and prevention efforts to combat the ongoing HPAI panzootic.
Further Reading:
- “Tackling avian influenza with automated detection for an early warning system,” Western Ecological Research Center (WERC), United States Geological Survey (USGS)
- “More avian flu outbreaks in Pennsylvania, Colorado,” Stephanie Soucheray, CIDRAP
- “Bird flu kills 10th dolphin in Florida’s Indian River Lagoon,” Jim Waymer, Florida Today
Institute for Science and International Security Hosts Two-Day Course on Nuclear Non-Proliferation at George Mason University!
From ISIS: “The Institute for Science and International Security, a non-partisan NGO based in Washington D.C., focused on the non-proliferation of nuclear weapons, is bringing its free technical course on nuclear weapons and their proliferation to the GMU. David Albright and Sarah Burkhard will provide a solid foundation of the scientific and technical aspects of nuclear weapons, including the key facets of developing nuclear weapons, including fissile material production – uranium enrichment, plutonium production and separation – and nuclear weaponization and delivery. It will include how we have learned about secret nuclear weapons programs in Iran, Pakistan, North Korea, South Africa, and other countries.
The course will be taught in two half-day sessions, March 31st and April 6th, at George Mason University, Van Metre Hall, Fairfax Drive, Arlington, VA. Attendees will receive a certificate of attendance. The course is free, but registration is required by sending an email with your full name and affiliation to bitaraf@isis-online.org.”
Eighth Session of the BWC Working Group Convenes in Geneva
The Eighth Session of the Working Group (WG) on the strengthening of the 1972 Biological and Toxin Weapons Convention (BWC/BTWC) is scheduled to convene in Geneva from 9 to 13 February 2026. Proceedings for public meetings will be video-streamed via UN WebTV and audio-streamed via Listen Live. Official documents and other materials, including details of side events, are being posted by the BWC Implementation Support Unit (ISU) to the official web page of the Eighth Session, which can be found here. A consolidated collection of Richard Guthrie’s reporting on the BWC meetings is available online: https://www.cbw-events.org.uk/bwc-rep.html
In Other News
Global Health Governance and US WHO Relations
- “China criticizes U.S. for WHO pullout, accusing it of sidestepping international law,” Helen Branswell, StatNews
- “New York City Partners with W.H.O. as U.S. Withdraws From Global Effort,” Joseph Goldstein, NYTimes
- “Pandemic security needs national leadership,” Maria D. Van Kerkhove and Chikwe Ihekweazu, Science
- “Biodefense Blind Spot: Why Washington Confuses Pandemics with Bioweapons,” Junaid Nabi, War on the Rocks
- “One Year Post-USAID, Global Health Funding Stuck in Limbo,” Allison Krugman, Think Global Health
- “The Great Aid Recession: 2025’s Humanitarian Crash in Nine Charts,” Sam Vigersky, CFR
- “DHS warns of increasing trend in domestic partner poisonings,” Aaron Katersky and Josh Margolin, ABC News
US Biodefense and Domestic Preparedness
- “How Donald Trump Can Fix US Biodefense,” Robert C. O’Brien, The National Interest
- “Respiratory PPE as Day-Zero Defense Against Biothreats,” Eva Siegmann, Council on Strategic Risks
- “Strengthening biosecurity and biosafety oversight: CEPI publishes its first Biosecurity Policy,” CEPI
- “Biosafety and Biosecurity: Comparing the U.S. and Selected G20 Members,” GAO
Public Health Infrastructure and Trust
- “Poll: Trust and Confidence in the CDC Remain at Low Point After Changes to Recommended Childhood Vaccines; More Say the Changes Will Hurt than Help Children’s Health,” KFF
- “A ‘shadow CDC’ is scrambling to fill gaps in public health data,” Lauren J. Young, Edited by Tanya Lewis, Scientific American
- “Newly revealed emails undermine RFK Jr testimony about 2019 Samoa trip ahead of measles outbreak,” Michelle R Smith and Ali Swenson, The Guardian
- “New Poll: 9 in 10 Americans Say Policymakers Must Protect Vaccine Access,” Partnership to Fight Infectious Disease
- “4 times as many measles cases in a few weeks than US typically averages in a whole year: CDC,” Youri Benadjaoud, ABC News
Science Workforce & Research Funding
- “U.S. government has lost more than 10,000 STEM Ph.D.s since Trump took office,” Monica Hersher, Jeffrey Mervis
- “Trump tried to gut science research funding. Courts and Congress have rebuffed him,” Evan Bush, NBC News

NEW: Atlantic Council’s Pick Your Poison: The Enduring Threat of Biological Toxins
GMU’s Biodefense Director Gregory Koblentz will serve as a panelist at the Atlantic Council’s Bipartisan Commission on Biodefense latest meeting, mapping the threat picture of biological toxins and its implications for future biological attacks. He will be speaking on the spectrum of toxin threats, including foodborne exposure, state-sponsored assassination, terrorism, synthetic production, and warfare. This meeting of the Commission will be focused on ease of availability and control of ricin, botulinum, and other toxins, making them attractive weapons for use by U.S. adversaries, and the unique scientific challenges inherent in detecting, characterizing, and attributing toxin attacks. The discussions will also touch upon the vulnerability of food and agricultural systems to toxin threats and the measures required to secure the supply chain from intentional adulteration.”
This event will take place virtually on Thursday, February 26, at 10:00 AM ET. Learn more and register here.
NEW: World Changing Innovations in Science & Technology Summit
From the National Institute for Defense Health Cooperation (NIDHC): “The National Institute for Defense Health Cooperation (NIDHC), a component of the Uniformed Services University that supports the Department of War and other federal partners, will host the World Changing Innovations in Science and Technology Summit. The S&T Summit is organized into four modules, focused on key S&T domains: biotechnology, advanced computing, space technology, and nuclear energy. Our Nation’s leading subject matter experts from government, academia, industry, and federally funded laboratories will present on cutting-edge developments and emerging risks from a medical and public health perspective.
Attendees are welcomed from Federal, state, and local governments, industry, academia, and other non-governmental organizations to explore emerging innovations in science and technology, assess their potential threats and opportunities.
This event will take place from March 9-20, in Bethesda, Maryland. Learn more and register here.
The 100 Days Mission: Defending Against Pandemic and Biological Risks in a Fragmented World
From Foreign Policy: “The 100 Days Mission—led by CEPI and endorsed by G7 and G20 leaders—is establishing tools and capabilities to ensure that safe and effective vaccines can be developed within 100 days of a new viral threat. What will it take to embed these capabilities into national security strategies, defense planning, and sustained financing—before the next crisis tests the system? Held against the backdrop of the 2026 Munich Security Conference, Foreign Policy and CEPI will host an official side event to examine how cross-sector partnerships, shared planning, and coordinated financing can close preparedness gaps and strengthen collective defenses against future biological threats.”
This event will be held in-person in Munich, Germany, on February 14 from 9:00 – 10:30 am CET. Learn more and register here.
EBSA – Cyberbiosecurity: A Unique Marriage of Biosecurity and Information Systems
From EBSA: “Increasing reliance on the internet and technology in research and biomedical laboratories has opened these organizations up to increased focus for cyberattacks. These attacks may be perpetrated by external actors or individuals internal to the organization (purposefully or accidentally) and can take advantage of a lack of knowledge by research staff about phishing, access control best practices, and network security especially of research instrumentation, automated laboratory equipment, and building automation and control systems that may be connected to the internet/cloud. This talk will introduce the types of cyberthreats that have been commonly perpetrated against research and biomedical organizations. We will discuss existing guidance documents from the National Institute of Standards and Technology (NIST) and the International Organization for Standardization (ISO) that address critical infrastructure cybersecurity and information systems security controls and how they can be applied to all biomedical research organizations (BSL-1 through BSL-4). Finally, we will explain some of the best practices described in these documents relating to identity management and access control, awareness and training, and data security to give biorisk management and laboratory personnel a better working understanding of cyberbiosecurity.”
This event will take place virtually on Thursday, February 19 from 14:00 – 16:00 CET. Learn more and register here.
Public Health on the Pitch: Radiation Readiness for the FIFA World Cup
From ASTHO: “Join ASTHO for a webinar focused on public health preparedness and response related to the upcoming FIFA World Cup. This session will highlight response operations to a radiological incident during a mass gathering. The discussion will be led by Dr. Ziad Kazzi, an emergency physician and medical toxicologist with internationally recognized expertise in poisoning and radiation toxicology.
Dr. Kazzi will address key response considerations from a health department and public health lens, including triage, contamination management, radiation safety roles and detection assets, community reception centers, medical countermeasures, and more. There will be time for a Q&A session to address questions from attendees.”
This event will take place virtually on February 24 from 1:00 – 2:00 PM ET. Learn more and register here.
Johns Hopkins H5N1 Influenza Preparedness & Response Forum
From Johns Hopkins: “Join us in person for the Johns Hopkins H5N1 Influenza Preparedness & Response Forum! This Forum convenes state and local public health officials, federal agency representatives, Congressional staff, veterinarians, diagnosticians, and leading scientific researchers to address the urgent challenges posed by H5N1 and future influenza threats. H5N1 already poses a major threat to the nation’s livestock and poultry industries. If it were to acquire the ability to spread from person to person, there would be increased challenges for detection, containment, and response. This Forum will focus on actionable, science-based recommendations and foster cross-sectoral collaboration to strengthen preparedness and response capacity across the One Health spectrum.”
This event will take place in Washington, DC, on Tuesday, March 3 from 9:00 AM-5:00 PM ET. Learn more and register here.
Nexus Series: AIxBio: Workshop 2 – Strategies for Responding to Exponential AI and Biotechnology Growth
From AI for SynBio: “Background: The ever-increasing acceleration of agentic artificial intelligence (AI) and biological design tools has transformed the technological landscape, enabling tremendous benefits and potential misuse that could massively impact national security and public health. Mitigating this risk will require collaboration across Government, Industry, and Academia with both technical and policy focus. Significant effort has already been made to raise awareness of this challenge, but additional discussion is necessary to maintain pace with the speed of evolving technology. The second workshop in this series will build upon insights from our first workshop and take place over two days.”
This event will take place in Washington, DC, on March 4-5, 2026. Learn more and RSVP here.
CBRNe Convergence Canada 2026
CBRNe World is hosting its fourth CBRNe Convergence Canada event this year in Toronto! This event will focus on a range of topical issues, including responding to potential CBRN incidents in the High North, response to terrorist attacks at major sporting events, and presentations on Canadian response capabilities, and the largest CBRN/Hazmat exhibition in Canada.
This event will take place April 13-15 at the Double Tree by Hilton Hotel, Toronto Downtown. Learn more here, and stay tuned for forthcoming information about CBRNe Convergence this November in Knoxville, TN!
International Conference CBRNe Research & Innovation
From CBRNE: “The last 40 years have demonstrated that both military and civilian populations could be exposed to highly hazardous CBRNE agents following conflicts, natural outbreaks and disasters, industrial incidents or terrorist attacks. Worldwide, researchers, responders and industrial capacities have been commited to provide adapted response to these challenges. The CBRNE Research & Innovation Conference includes workshops and demonstrations of innovative materials, technologies and procedures, according to the following themes: Detection (identification), Protection (decontamination, medical countermeasures), and risk & crisis management.
This event will take place in Arcachon, France, from May 19 – 21, 2026. Learn more and RSVP here.
GHS 2026
From GHS: “We’re excited to officially announce that the 4th Global Health Security Conference (GHS2026) will be held in Kuala Lumpur on the 9 – 12 June, 2026!”
“Building on the incredible momentum of GHS2024 in Sydney, we look forward to bringing together the global health security community once again – this time in one of Southeast Asia’s most vibrant and dynamic cities.”
“Registration and Call for Abstracts are now live!”
Learn more, submit abstracts, and register here.

NEW: 9th World One Health Congress – Calls for Abstracts
From Global One Health Community: “The One Health Congress invites abstracts, session concept notes, and workshop proposals addressing innovative research, policy, and practice across human-animal-plant-environment interfaces. Submissions are welcomed under the following themes: 1) Climate change, environment, and ecosystems health; 2) Biodiversity, wildlife, and pathogen interfaces; 3) Food systems, agriculture, and plant health; 4) Pathogens, microbiome, and intervention strategies; 5) Antimicrobial resistance (AMR); 6) Data, technology and innovative analytics; 7) Knowledge, education and communities; 8) Governance, policy and legal frameworks, 9) One Health implementation and financing; 10) Preparedness, resilience and economic evaluation; 11) Biosecurity, biosafety and global health security; 12) The critical role of the private sector in One Health policy.
Learn more and submit here.
NEW: Far-UVC Program Officer at Blueprint Biosecurity – Job Opening
From Blueprint Biosecurity: “Blueprint Biosecurity is seeking a full-time Far-UVC Program Officer to be a driving force behind our far-UVC program. As Far-UVC Program Officer, you will help address these research gaps to transform early-stage potential into deployable protection that could save lives during the next pandemic. Reporting directly to the Built Environment Program Director, this role will shape and execute our far-UVC strategy, with a particular emphasis on directing research and funding to advance far-UVC science and technology, and communicating our work to partners, experts, and the general public.”
The deadline to submit applications is February 20. Learn more and submit your application here.
NEW: The Youth for Biosecurity Initiative 2026 – Applications Open!
From the United Nations: “The United Nations Office for Disarmament Affairs (UNODA) in Geneva is delighted to launch a call for applications to the Fourth Edition of the Youth for Biosecurity Fellowship.
Since its establishment in 2019, the programme has engaged over 140 young scientists through an innovative and engaging training course. 20 competitively selected young scientists from the Global South will take part in a series of interactive webinars prior to an in-person visit during the meeting of the BWC Working Group on the Strengthening of the Convention in Geneva in August 2026.
Please note all successful applicants will be responsible for attaining any necessary travel documents to attend the in-person visit in Switzerland, including visas. Travel tickets in economy class and a daily subsistence allowance will be provided to successful applicants for the official dates of the trip in line with UN rules and regulations.
The deadline for applications is March 5. Learn more and apply here.
One Health Commission Board of Directors – Call for Applications
From OHC: “The One Health Commission (OHC) is pleased to announce an open call for board members to join our governing body. As we implement our new constitution and bylaws, we are seeking distinguished experts who embody collaborative, multi- and transdisciplinary approaches for addressing shared health challenges across human, animal, environmental, and plant health domains.”
The deadline to submit applications is February 15. Learn more and submit your application here.
OPCW Senior Science Policy Officer (AI, Data Sciences and Knowledge Management) (P-4) – Job Opening
From OPCW: “The Office of Strategy and Policy (OSP) develops the Technical Secretariat’s strategic planning and provides policy advice to the Director-General and the Secretariat’s management, particularly relating to cross-cutting issues such as incidents of chemical weapons use, non-routine missions, chemical security, engagement with international organisations and chemical industry, education and outreach, counter-terrorism, and science and technology. This role in OSP ensures that the Science Policy Adviser is provided with expert advice on advancements in science and technology, with a focus on artificial intelligence (AI) and digital sciences, through continuous monitoring and provision of specialised advice on applicability to the work of the OPCW. The individual will also help lead, coordinate, and integrate AI and data science-driven approaches and solutions to and in the work of the Secretariat. This role will also take the lead on the Organisation’s knowledge management activities, with a focus on the development and implementation of knowledge management systems and the development and application of digital tools to knowledge management processes.”
The deadline to apply is February 18. Learn more and submit your application here.
ICGEB Fellowships for Scientists in Biosecurity
From the International Centre for Genetic Engineering and Biotechnology: “With the co-funding of the Norwegian Agency for Development Cooperation through the United Nations Office for Disarmament Affairs (UNODA), BWC ISU, the ICGEB offers early-career scientists from OECD/DAC countries a fellowship at ICGEB laboratories in Italy, India, South Africa and at the ICGEB Regional Research Centre in China, to receive hands on training in life sciences and detection and response to biological threats.”
The closing date for applications is March 31, 23:59 CET. Learn more and apply here.