By Chris Healey
An Ebola hemorrhagic fever outbreak in the Republic of Guinea has raised concerns about the illness and its spread to countries outside Africa.
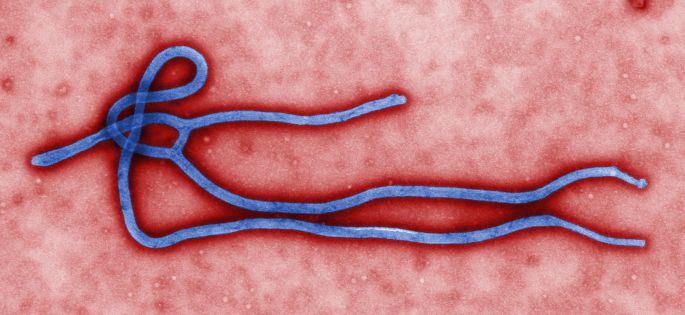
Ebola is a virus in the filoviridae family. Of the five Ebola species, only Zaire, Sudan and Bundibugyo species have caused outbreaks in humans.
Ebola appears to be an incidental host of humans from a natural cycle involving bats and nonhuman primates. Humans enter the cycle after contact with blood and tissue from nonhuman primates, often after instances of butchering and animal cruelty. Transmission routes from bats to humans are possible but currently unknown.
The first confirmed outbreak of Ebola occurred in 1976 in Nzara, a small town in southern Sudan. Workers from a local cotton factory spread the disease to their relatives and others in the community. The outbreak lasted several months. Of the 284 cases in that outbreak, 151 died.
Ebola causes Ebola hemorrhagic fever, a severely debilitating illness affecting multiple organ systems. Ironically, hemorrhage is an uncommon symptom reported in fewer than half of all cases. Death typically occurs due to shock from fluid loss and organ failure.
Mortality rate is species dependent. Bundibugyo has a 36% fatality rate, Sudan 55%, and Zaire 90%. There is no cure, but administration of an experimental vaccine that targeted a viral protein used for attachment and entry to the host cell was attributed to the survival of an accidentally-exposed laboratory researcher in Germany.
Ebola has been the inspiration for fictional literature and film. The Hot Zone by Richard Preston provides an overview of Ebola and other filoviruses before focusing on the discovery of Reston Ebola, a species known to only sicken nonhuman primates, in Reston, Virginia. The 1995 movie Outbreak featured an Ebola-like virus as the primary plot device.
Both works exaggerate Ebola hemorrhagic fever’s morbidity and hemorrhagic symptoms to inflate pathogenicity and pandemic potential. While there is no denying the severity of Ebola hemorrhagic fever illness, it is an ineffective cause of pandemics.
Perhaps the most misunderstood characteristic of Ebola is its method of transmission. Direct physical contact with infected persons or their bodily fluids is required to transmit the illness. There is no evidence of airborne transmission among Ebola species known to affect humans. Ebola rapidly becomes nonviable outside the host when aerosolized in uncontrolled, non-laboratory settings.
Ebola has an incubation period of 4 to 10 days. Rapid onset allows healthcare providers to quickly identify affected individuals. Only symptomatic individuals can spread the disease. In other words, the disease cannot be transmitted during incubation or through non-symptomatic cases. A short, non-communicable incubation period limits Ebola’s pandemic potential.
Limitations of direct person-to-person spread can assist public health response efforts in the event of an Ebola hemorrhagic fever outbreak. Quarantine procedures are effective in restricting the illness shortly after detection. Due to the lack of airborne transmission, direct contacts with sickened individuals can be traced and contained, arresting illness progression. Ebola hemorrhagic fever rarely escalates past a localized outbreak if adequate public health infrastructure is present.
Media consumers should be wary of fictional or exaggerated portrayals of illness. The media’s tendency to misuse and dramatize information can breed undue fear in the event of a public health crisis.