This week’s Pandora Report covers news from the Biodefense Graduate Program, the winners of the 2024 OPCW-The Hague Award, reports of a mysterious illness in the DRC, and much more.
Biodefense Doctoral Student Selected for Next Generation Leader Program
First-year Biodefense PhD student Katie Dammer was recently selected for the Next Generation Leaders program as part of the Spirit of Asilomar conference that will be held in February 2025. “The Spirit of Asilomar and the Future of Biotechnology” summit will occur on the 50th anniversary of the 1975 international meeting on recombinant DNA, where scientists discussed the hazards and benefits of emerging biotechnology and voluntarily agreed to set new standards for the regulation of biohazards. The 2025 iteration of this summit will focus on artificial intelligence, synthetic biology, pathogen research, and other related topics. Dammer currently is a Biosecurity Fellow at the Horizon Institute for Public Service working as the Special Assistant for Global Health Security & Biodefense at the National Security Council.
2024 OPCW-The Hague Award Recipients Announced
Last month, OPCW Director-General Amb. Fernando Arias and The Hague Mayor Jan van Zanen announced the winners of this year’s OPCW-The Hague award: Alegeria’s National Institute of Criminalistics and Criminology of the National Gendarmerie (NICC/NG) and the Indian Chemical Council (ICC). NICC/NG is “a forensic science institute focused on advancing crime-fighting capabilities by integrating scientific methods into judicial and criminal processes.” The ICC “is a chemical industry body recognized for its role in promoting chemical safety, compliance with the Convention, and enhancing industry-wide security practices in India.” Read more about the recipients and this year’s award selection here.
DRC Reports Outbreak of Unknown Flu-Like Illness
The Democratic Republic of Congo (DRC), Africa CDC, and WHO are investigating an outbreak of an unknown, influenza-like disease in Kwango province that has infected at least 376 people and killed at least 67 (though some outlets report 143 deaths) since late October. Africa CDC reports that symptoms include fever, headache, cough, difficulties breathing, and anemia. Children under five are the most affected group, accounting for more than half of all cases and the majority of deaths. The outbreak started in the Panzi Health Zone, a remote part of the province. Officials report that they did not learn of the outbreak until six weeks after it began. In better news, the WHO has confirmed that the DRC’s mpox outbreak appears to be “plateauing”.
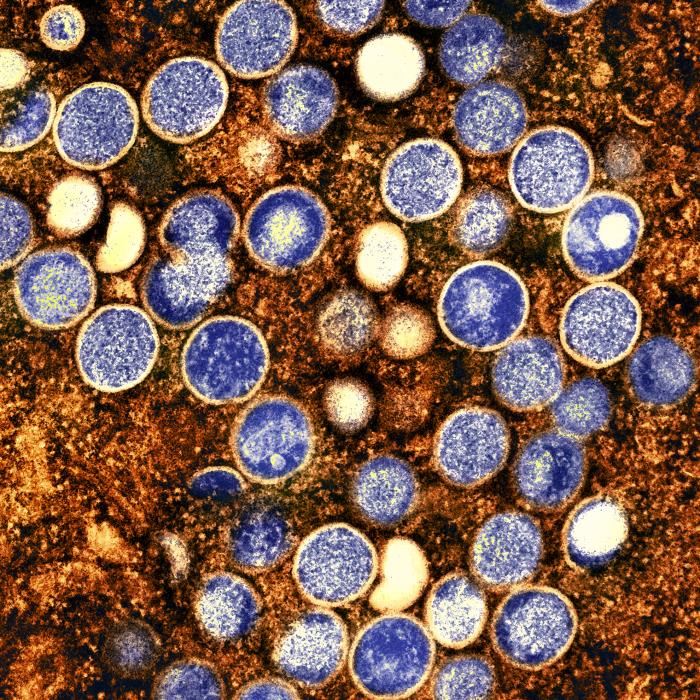
Raw Milk Recalled in California Following Bird Flu Detection
In a predictable turn of events, a farm in California has made a voluntary recall of its products and halted production after samples of its products tested positive for avian influenza. The recall has expanded since the initial recall of two lots of product on November 21. The California Department of Food and Agriculture has quarantined the farm and suspended the distribution of raw milk, cream, kefir, butter, and cheese products produced on or after November 27. The farm in question, Raw Farm of Fresno, has gained popularity with fans of social media “health influencers” and HHS Secretary nominee RFK Jr.. It frequently sells its products in natural supermarket chains like Sprouts Farmers Market.
The brand fell under FDA and CDC scrutiny earlier this year amid E. coli concerns, though the company insists it was the victim of “severe bias” from the agencies. Mark McAfee, CEO of Raw Farm, is insisting now that the actions taken by the state are politically-driven, despite samples of his company’s products testing positive for the virus. McAfee appears to have been encouraged by RFK Jr. to apply for a position at the FDA as the “FDA advisor on raw milk policy and standards development.”
Further Reading:
- “A Bid Flu Pandemic Would Be One of the Most Foreseeable Catastrophes in History,” Zeynep Tufecki, The New York Times
- “Bird Flu, Explained,” David Leonhardt, The New York Times
House Select Subcommittee on the Coronavirus Pandemic Releases Final Report
The House Select Subcommittee on the Coronavirus Pandemic recently published its final report, “AFTER ACTION REVIEW OF THE COVID-19 PANDEMIC: The Lessons Learned and a Path Forward”. The more than 500-page document covers a variety of topics, including vaccines, use of pandemic relief funds, and public health guidance. The report begins with the claim that SARS-CoV-2 “likely emerged because of a laboratory or research related accident,” despite the Intelligence Community remaining split on the consensus and many in the scientific community disagreeing. The report is also critical of mitigation efforts like face masking and social distancing, though it praises travel restrictions. The report also focuses its attention heavily on the EcoHealth Alliance.
The Select Subcommittee’s minority released its own final report. A spokesperson for the minority said in a statement, “Select Subcommittee Republicans’ final report reflects two years wasted on political stunts instead of preventing and preparing for the next pandemic…Instead of coming together with Democrats to get ahead of future viruses or fortify America’s public health infrastructure and workforce, Select Subcommittee Republicans prioritized extreme probes that vilified our nation’s scientists and public health officials in an effort to whitewash former President Trump’s disastrous COVID-19 response.”
The minority report blasts the majority’s criticism and targeting of former NIAID Director Anthony Fauci as “baseless and frivolous” and explains that “Today, a zoonotic origin and lab accident are both plausible, as is a ‘hybrid’ scenario reflecting a mixture of the two…It was repeatedly explained to the Select Subcommittee that all prior epidemics and pandemics, as well as almost all prior outbreaks, have zoonotic origins. At the same time, a lab origin for SARS-CoV-2 also remains plausible.”
Further Reading: “Sick Animals Suggest COVID Pandemic Started in Wuhan Market,” Smriti Mallapaty, Nature
Trump Continues to Make Controversial Administration Selections
Trump Picks Jay Bhattacharya to Lead NIH
Late last month, President-Elect Trump announced Jay Bhattacharya, a Stanford-trained physician and economist, as his pick to lead the NIH. Bhattacharya gained national attention for co-authoring the October 2020 letter known as the Great Barrington Declaration, which called for the rollback of pandemic-related shutdowns, drawing rebuke from then NIH director Francis S. Collins. Bhattacharya also was among several academics who met with Trump in August of 2020, claiming that the pandemic was not as severe as public health officials had warned. The letter gained broader criticism for its focus on herd immunity, especially as COVID-19 vaccines were not available at that point, so relying on herd immunity would lead to even more unnecessary illnesses and deaths.
Trump Picks Jim O’Neill for HHS Deputy Secretary
Trump also announced in late November that he will select Jim O’Neill, a Silicon Valley investor and former federal health official, as his selection to be Deputy Secretary at HHS. O’Neill joined HHS in 2002, holding several roles throughout his tenure, including serving as a top aide to the then-deputy secretary of the department. He then moved on to Silicon Valley, becoming a close ally of Peter Thiel, a close adviser to Trump during his first term who has also long championed VP-Elect JD Vance.
Trump Announces Dave Weldon as CDC Director Pick
In an unforeseen move, Trump picked former Congressman Dave Weldon as his nominee to lead the Centers for Disease Control and Prevention. Weldon has been out of politics for the last fifteen years, running a private medical practice in Florida. During his tenure in Congress, he made controversial statements about the safety of MMR and and HPV vaccines. His views have often aligned with those of RFK Jr., whom he has been friends with for more than two decades. Weldon has said of his time in Congress that he worked with Kennedy “to get the mercury out of the childhood vaccines.”
Further Reading:
- “Former Trump FDA Chief is Seeking to Undermine RFK Jr.’s Senate Confirmation,” Rachel Cohrs Zhang, STAT News
- “Long a ‘Crown Jewel’ of Government, N.I.H. Is Now a Target,” Teddy Rosenbluth and Emily Anthes, The New York Times
- “US Membership in the World Health Organization is On the Line with Trump’s Return,” Carmen Paun, Politico
- “How Measles, Whooping Cough, and Worse Could Roar Back on RFK Jr.’s Watch,” Arthur Allen, KFF Health News
- “Science Could Solve Some of the World’s Biggest Problems. Why Aren’t Governments Using It?” Helen Pearson, Nature
White Helmets Urge International Community to Take Action to Protect Syrian Civilians from Chemical Warfare
The Director of the White Helmets, Raed Al-Saleh, warned recently that Syria’s authoritarian president, Bashar Al-Assad, could very soon use CW against civilians in an effort to stop rebels advancing in the northwest of the country. In a statement, Al-Saleh said “”For six days now, as the map of military control has changed, brutal attacks launched by the Syrian regime, Russia, and Iranian cross-border militias on Syrians have escalated especially in areas outside their control in northwest Syria…I am gravely concerned about the lives of every Syrian because of the real threat of chemical attacks.”

“Strategic Report on Research and Development in Biotechnology for Defense Innovation”
From NASEM: “At the request of the National Security Commission on Emerging Biotechnology, Strategic Report on Research and Development in Biotechnology for Defense Innovation provides an overview of the current landscape of artificial intelligence and machine learning (AI/ML)-enabled biotechnology, the opportunities it presents, and the challenges it poses. This report offers a strategic vision for connecting scientists and technologists to build on, leverage, and tailor advances at the intersection of AI/ML, automated experimentation, and biotechnology to drive innovation in defense-related biotechnologies. Strategic Report on Research and Development in Biotechnology for Defense Innovation makes recommendations to address long-standing challenges that have limited research, development, prototyping, testing and evaluation, and eventual use of biotechnologies. Addressing these challenges will help to advance U.S. national security and defense by improving the performance of existing capabilities, enabling the creation of domestic supply chains of valuable products, reducing reliance on processes and chemicals that are harmful to the environment, and/or adding new capabilities not currently possible with established technologies.”
“Anticipating Biological Risk: A Toolkit for Strategic Biosecurity Policy”
Stephen Batalis for CSET: “Artificial intelligence (AI) tools pose exciting possibilities to advance scientific, biomedical, and public health research. At the same time, these tools have raised concerns about their potential to contribute to biological threats, like those from pathogens and toxins. This report describes pathways that result in biological harm, with or without AI, and a range of governance tools and mitigation measures to address them.”
“Strengthening the Biological Weapons Convention”
Jez Littlewood and Filippa Lentzos recently published this piece with the Arms Control Association discussing the BWC working group and its efforts to improve the BWC. They write in part, “Substantial progress has been made in some areas, but beneath the surface is a broader conflict about the shape of arms control agreements generally. This raises a question about whether strengthening the BWC needs to follow the traditional model of legally binding multilateral agreements with declarations, inspections, investigations, and an international organization where consensus rules or whether states-parties can agree to a new model that allows states to opt in to the mechanisms with which they agree and opt out of any processes or new commitments they are unable to support.”
“BWC at 50: Taking Bold Steps to Secure the Future”
Gabrielle Essix recently authored this rundown on the BWC’s successes, shortcomings, and future for NTI| bio. She writes in part, “As we look ahead to the future of the BWC, the role of civil society will become increasingly critical. Organizations like NTI provide a bridge between governments, scientists, and the public, ensuring that biosecurity remains a global priority. By advocating for stronger international norms and pushing for innovative solutions to new challenges, NTI can help make the BWC an effective tool in the fight against the development and use of biological weapons.”
“Possible Models of BWC Verification”
James Revill authored this brief for UNIDIR: “This briefing serves as a primer for consideration of possible models of verification. Past discussions of verification in the Biological Weapons Conference (BWC) have largely focused on the development of a more traditional disarmament verification regime, akin to the model established in the Chemical Weapons Convention (CWC) and envisaged in the BWC Protocol Negotiations. Such a model is often considered the standard model for verification and could provide greater confidence in compliance with the BWC.”
“However, the traditional model of verification is not the only model available to BWC States Parties. Depending on the function(s) and focus of any verification mechanism, other options could be developed for BWC verification that might more effectively address the concerns of BWC States Parties and potentially reduce costs of verification while still increasing confidence in compliance.”
BioWeapons Prevention Project: “The Closure of the Fourth Sessions Preparations for the Fifth”
From BWPP: “The Working Group (WG) on the strengthening of the 1972 Biological and Toxin Weapons Convention (BWC/BTWC), will convene for its Fifth Session on 2 December having finished the Fourth Session on 23 August. The topics for discussion in the WG were decided at Ninth BWC Review Conference, held in 2022. The two-week Fifth Session will be followed by the annual Meeting of States Parties (MSP) which is scheduled for 16-18 December…This report focuses on some overarching issues. Individual topics up for discussion during the Fifth Session have been examined in earlier reports in this series, and in particular in the ‘setting the scene’ reports.”
Read more here.
“Biocrimes: Safeguarding Clinical and Public Health Microbiology Labs Against Insider Threats”
Casey Shroeder authored this piece for Lab Manager, writing in part “Within clinical and public health microbiology laboratories where scientists work to diagnose infections and/or protect public health, the potential for biocrimes and insider threats is a serious risk that is often overlooked. These laboratories, which handle not only routine human pathogens but also antimicrobial resistant strains, emerging pathogens, and potential biothreat pathogens, must remain vigilant against those who might exploit their access for malicious purposes.”
“Bacteriologic and Genomic Investigation of Bacillus anthracis Isolated from World War II Site, China”
Wu et al. recently published this article in Emerging Infectious Diseases: “Records suggest Bacillus anthracis was used in biowarfare during World War II, but evidence remains limited. We isolated B. anthracis from soil at the remains of a World War II–era laboratory in China. Phenotypic and genomic analyses confirmed the finding, highlighting the value of microbial forensics in biothreat investigation.”
“Modern Warfare is Breeding Deadly Superbugs. Why?”
Francesca Mari recently published this piece in The New York Times Magazine, explaining in part “By 2050, The Lancet predicts that antimicrobial resistance will kill 8.22 million people per year, more than the number currently killed by cancer. (For context, Covid claimed an estimated three million lives during all of 2020.) And a growing body of research suggests that the 21st-century way of warfare has become a major driver of that spread. Nations of the Middle East, like Iraq, Syria, Yemen and Afghanistan, now suffer from particularly high rates of multidrug-resistant pathogens, and some of the world’s most fearsome superbugs have incubated in the region — Klebsiella pneumoniae, Pseudomonas aeruginosa, E. coli, MRSA and perhaps most notably A. baumannii, a strain of Acinetobacter that traveled home with U.S. soldiers, where it became nicknamed “Iraqibacter.”’
“Global Report on Infection Prevention and Control 2024”
From WHO: “Health care-associated infections (HAIs) affect patients and health systems every day, causing immense suffering, driving higher health-care costs and hampering efforts to achieve high-quality care for all. HAIs are often difficult to treat, are the major driver of antimicrobial resistance (AMR) and cause premature deaths and disability. The COVID-19 pandemic, as well as outbreaks of Ebola, Marburg and mpox are the most dramatic demonstrations of how pathogens can spread rapidly and be amplified in health care settings. But HAIs are a daily threat in every hospital and clinic, not only during epidemics and pandemics. Lack of water, sanitation and hygiene (WASH) in health care settings not only affects the application of infection prevention and control (IPC) best practices but also equity and dignity among both those providing and receiving care. However, there is strong evidence that a large proportion of these infections could be prevented with IPC measures and basic WASH services, with a high return on investment. This second global report on IPC provides updated evidence on the harm caused to patients and health workers by HAIs and AMR, and presents an updated global analysis of the implementation of IPC programmes at the national and health care facility levels across all WHO regions. “
Bloomberg FOIA Files: Kremlin Targeting Putin’s Political Adversaries, Has Ability to Assassinate Targets with Chemical and Biological Weapons
In this edition of Bloomberg News’ FOIA Files, Jason Leopold discusses a recently-released memo from ODNI discussing targeting killings of Vladimir Putin’s political adversaries, and the means by which the Russian state is able to accomplish this. In the memo, intelligence officials assessed that “Russia has the capability to assassinate individuals using chemical and biological agents,” and that they have the means to track dissidents and defectors. The memo also explains that “The development of chemical or biological agents with lower risk of attribution might tempt the Kremlin to consider assassinating individuals,” in addition to discussing the death of Russian businessman Alexander Perepilichnyy, who was “reportedly assassinated with a biological toxin in the UK in 2012 shortly before he was scheduled to testify about a Kremlin tax fraud network.”
“Reviving Chemical Weapons Accountability in a Multipolar World”
The Center for Strategic and Strategic & International Studies published this commentary by Natasha Hall and Doreen Horschig ahead of the 29th Session of the Conference of States Parties of the Organization for the Prohibition of Chemical Weapons. In it, they discuss the strain the CWC has faced in the last decade and how its strength might be restored. They write in part, “Next week’s conference presents an opportunity to revitalize efforts toward accountability and global cooperation. The United States, in particular, is in a position to reverse course on the dangerous erosion of the chemical weapons norm and maintain the integrity of the CWC. But to do so, it will need to engage friend and foe alike.”
“The Islamic Republic’s Work on Pharmaceutical Based Agents”
This report was authored by Mohammadreza Giveh and the Good ISIS Team for the Institute for Science and International Security. “This report discusses multiple Iranian security complexes that have been preparing production of fentanyl and medetomidine based incapacitating and lethal agents. These complexes have been working on pillars of producing those weapons: large-scale cost-efficient synthesis of the compounds with maximum potency, evaluating a stable chemical mixture based on those agents that can be aerosolized using a propellant, and developing the delivery of the agents through grenades, bullets, or drones.”
“Chemical Weapons Disinformation in Ukraine”
From GP WMD Counter Disinfo, this series includes three briefs: “Understanding Russia’s Chemical Weapons Allegations in Ukraine,” “Selected Examples of CW Allegations and Related Disinformation Campaigns from the Russian Federation,” and “Strategy and Impacts of CW Disinformation”.
“Chemical Weapons Use in Ukraine Testa Global Norms to Breaking Point”
Lennie Phillips OBE and Gareth Williams discuss Russia’s use of CW in Ukraine and how it affects the CWC and OPCW in this RUSI piece, writing in part “A riot control agent found in samples collected from the confrontation lines in Ukraine by the Organisation for the Prohibition of Chemical Weapons implicates Russia in yet another breach of the Chemical Weapons Convention. But what steps can states parties to the convention take next?”
“Russia Fails to Make OPCW Executive Council for Second Year Running”
Patrick Norén discusses Russia’s failure to be elected to the OPCW’s Executive Council for the second year in a row in this piece for CBNW.
“How Might Large Language Models Aid Actors in Reaching the Competency Threshold Required to Carry Out a Chemical Attack?”
Stendall et al. recently published this article in The Nonproliferation Review: “Artificial intelligence is a rapidly growing field, increasingly driving innovation in the sciences. This is a double-edged sword, with the benefits of scientific discovery tempered by potential opportunities for weaponization and misuse. Specifically, the implications for chemical security and chemical weapons are becoming increasingly clear. This article analyzes how large language models (LLMs)—particularly chatbots and chemical LLM assistants—might enable actors to better reach the competency threshold for a chemical attack, via enhanced methods for the identification, production, and use of chemical weapons. This would be particularly relevant for those at the lower end of the capability spectrum, such as terrorist groups and rogue individuals. An important historical context is provided throughout the article, with chemical attacks of the past illuminating the dangerous consequences of an easier-to-achieve competency threshold. A counterargument is also provided, analyzing the factors that might still limit malicious actors, as well as a description of how LLMs might be used to combat chemical terrorism. The article then concludes with a short list of key policy and governance suggestions for mitigating the risks.”
Read or listen to CNS’ interview with Stendall on this article here.
“Chemical Terrorism: Assessment of U.S. Strategies in the Era of Great Power Competition”
From NASEM: “Domestic and foreign violent extremist organizations, or terrorist groups, have caused a greater amount of harm with chemical agents than with biological or radiological weapons. The United States capacity and capability to identify, prevent, counter, and respond adequately to chemical threats is established by the strategies, policies, and laws enacted across multiple levels of government. While the number of chemical terrorism incidents has risen and fallen over time, there is no empirical or analytical indication that the threat is disappearing. This report comes at a time when the nation’s highest-level strategies have shifted from focusing primarily on violent extremist organizations to focusing more on Great Power Competition. This shift in relative perceived threat and consequent prioritization will impact efforts against chemical terrorism, and in turn, affect funding priorities. Revised risk assessments are needed to reprioritize risks guided by new strategies, so that strategy-aligned budgets can be created. The report recommends weapons of mass destruction budgets be aligned with evolving priorities and incentivize activities that transition promising research to operations.”
“Nuclear Terrorism: Assessment of U.S. Strategies to Prevent, Counter, and Respond to Weapons of Mass Destruction”
From NASEM: “For nearly eight decades, the world has been navigating the dangers of the nuclear age. Despite Cold War tensions and the rise of global terrorism, nuclear weapons have not been used in conflict since Hiroshima and Nagasaki in 1945. Efforts such as strategic deterrence, arms control and non-proliferation agreements, and the U.S.-led global counterterrorism have helped to keep nuclear incidents at bay. However, the nation’s success to date in countering nuclear terrorism does not come with a guarantee, success often carries the risk that other challenges will siphon away attention and resources and can lead to the perception that the threat no longer exists.”
“This report found that U.S. efforts to counter nuclear or radiological terrorism are not keeping pace with the evolving threat landscape. The U.S. government should maintain a strategic focus and effort on combatting terrorism across the national security community in coordination with international partners, State, Local, Tribal and Territorial authorities, the National Laboratories, universities and colleges, and civil society. Developing and sustaining adequate nuclear incident response and recovery capabilities at the local and state levels will likely require significant new investments in resources and empowerment of local response from Federal Emergency Management Agency (FEMA), working with the Centers for Disease Control and Prevention, Environmental Protection Agency, Department of Energy, and National Institutes of Health.”
“Ecological Threat Report”
The Institute for Economics & Peace recently released the fifth edition of its Ecological Threat Report, ” which analyses ecological threats in 207 independent states and territories. The report covers 3,518 sub-national areas which account for 99.99 per cent of the world’s population. The ETR assesses threats relating to food insecurity, water risk, natural disasters, and demographic pressure…The research takes a multi-faceted approach by analysing ecological threats at the national, subnational, and city level, while also assessing the threats against societal resilience and levels of peace. Comparing ecological threats against societal resilience enables IEP to identify the global regions, countries, and subnational areas most at risk of an ecological disaster, both now and into the future.”
“Healthcare Cybersecurity: HHS Continues to Have Challenges as Lead Agency”
This snapshot from the Government Accountability Office discusses previous GAO findings about HHS’ performance in healthcare cybersecurity, explaining that HHS has yet to implement all of GAO’s recommendations to address its challenges in this area. It concludes that “Until HHS implements our prior recommendations related to improving cybersecurity, the department risks not being able to effectively carry out its lead agency responsibilities, resulting in potential adverse impact on healthcare providers and patient care.”
“Lebanon: A Conflict Particularly Destructive to Health Care”
The WHO recently released this news post explaining that more health workers and patients have been killed proportionally in Lebanon than in Ukraine and Gaza, with 47% of attacks on health care in the country proving fatal as of November 21-a higher percentage than in any active conflict today globally. Read more here.
What We’re Listening To 🎧
Grow Everything Biotech Podcast, 103. DNA of Defense: Alexander Titus on How NSCEB is Advancing Biotech for National Security Challenges
“Karl and Erum bring on Dr. Alexander Titus, a commissioner on the National Security Commission on Emerging Biotechnology, to explore the exciting and challenging intersections of biotechnology and policy. Alexander shares his experiences from his unique journey across academia, government, and industry, diving into the role of biosecurity, the potential of synthetic biology, and the emerging convergence of tech and bio. They discuss ambitious projects like de-extincting the woolly mammoth, advances in biodefense, and the impacts of AI on biotech innovation. It’s a conversation that sheds light on how cutting-edge biotech could shape the future and the necessary balance between innovation and ethical responsibility.”
Listen here.

NEW: How to Avoid Human-Made Pandemics
From the Asia Centre for Health Security: “Studying viruses that could potentially cause outbreaks is one of the most effective ways to reduce the risk of pandemics. However, this type of research—especially when it involves collecting samples from the field and manipulating pathogens—can unintentionally lead to a pandemic if not managed carefully. Dr Lentzos will discuss her findings from the Pathogen Project, which brought together an international taskforce of scientists, biosecurity and public health experts, ethicists, and civil society leaders to seek consensus on this question: Can we agree on ways to manage research that carries pandemic risk as safely, securely and responsibly as possible?”
This event will take place on January 23 at 8 pm (GMT +8:00) via Zoom. RSVP here.
NEW: Preparedness in Rural Communities: National and State/Local Perspectives and Plans
From Penn State: “The COVID-19 pandemic and recent hurricanes have thrust the preparedness of rural communities into the national spotlight. At the federal level, the Administration for Strategic Preparedness and Response and the Centers for Disease Control and Prevention have recently released national goals and plans for preparedness of rural communities. The overall objective of this virtual, 2-day mini-symposium is to identify opportunities in public health and agricultural preparedness and response in rural communities. The mini-symposium will focus upon national perspectives on Thursday, January 30 and the state/local perspectives on Friday, January 31. Speakers include representatives of the Administration for Strategic Preparedness and Response, the Department of Homeland Security, US Department of Agriculture, the USA Center for Rural Public Health Preparedness, and state/local leaders.”
This event will take place on January 30 and 31, from 11 am to 2 pm ET each day. Learn more and register here.
Enhancing the Resilience of Healthcare and Public Health Critical Infrastructure: A Workshop
From NASEM: “Healthcare and public health infrastructure, technology, and operations are rapidly changing and are increasingly interdependent and interconnected. Threats to the nation’s critical social and physical infrastructure systems are also rapidly evolving and highly complex—posing potentially new or growing risks of disruption and challenging the assumptions used to design and protect these systems.”
“The National Academies Forum on Medical and Public Health Preparedness for Disasters and Emergencies will host a hybrid public workshop to explore strategies, policies, and innovative actions to improve the resilience of healthcare and public health (HPH) critical infrastructure to impacts from disasters and other emergencies.”
This event will take place in DC on December 9 and 10. Register here.
A New Paradigm for Threat Agnostic Biodetection: Biological Intelligence (BIOINT)
From PNNL: “Please join us in welcoming Swati Sureka, Strategy and Policy Analyst at Arctic Slope Regional Corporation (ASRC) Federal, where she supports the Office of the Deputy Assistant Secretary of Defense for Health Readiness Policy and Oversight. Her talk, titled “A New Paradigm for Threat Agnostic Biodetection: Biological Intelligence (BIOINT),” will take place on Tuesday, December 10th, at noon PT.”
Learn more and RSVP here.
Resilience in the Face of Global Risks
From CSR: “The Council on Strategic Risks (CSR) cordially invites you to our first annual CSR symposium, Resilience in the Face of Global Risks, scheduled for Tuesday, December 10, 2024. This is the first event bringing together all three of CSR’s institutions—the Center for Climate & Security, the Converging Risks Lab, and the Janne E. Nolan Center on Strategic Weapons—to engage with leaders across our mission sets.”
“The United States and its allies face a complex global security landscape where systemic risks like climate and ecological crisis, nuclear proliferation, and biological threats are blending with war, geopolitical competition, and human insecurity in new and profound ways. While there has been tremendous leadership—and meaningful progress—across these overlapping risk areas, they require persistent innovation in community building to meet the modern risk landscape.”
“The symposium will dive into the progress our communities have made—and how our passionate communities can better work together to help shape the future.”
“In addition to a keynote address, guests will hear from panels, take part in breakout sessions showcasing important issues and new ideas, plus engage with thought leaders between sessions. It will be a widely attended gathering, free to attendees.”
“As we enter a new year with an exceptionally dynamic security environment, we hope CSR’s December event will inspire and build a stronger community across professionals dedicated to anticipating and addressing the world’s greatest risks. We appreciate your consideration and hope you will be able to join us on the 10th of December.”
RSVP here.
Cyberbiosecurity Summit
From Johns Hopkins APL and Bio-ISAC: “Advancements in biomanufacturing and biotechnology drive the science we need to thrive, everything from apples to vaccines. The Cyberbiosecurity Summit 2025 convenes leading experts in biotechnology, biosecurity, and cybersecurity to explore the intersection of these fields and discuss the strategies to create a safe, secure future for us all.”
This event will take place February 25-26 in Laurel, MD. Register here and review the call for sessions here (closes 12/12).

NEW: Call for Experts, Standing Committee on Emerging Infectious Diseases and 21st Century Health Threats
“The National Academies is seeking suggestions for experts to be considered for the membership rotation or other engagement with the Standing Committee on Emerging Infectious Diseases and 21st Century Health Threats. The group will help inform the U.S. Department of Health and Human Services (HHS) Administration for Strategic Preparedness and Response (ASPR) on critical science and policy issues related to emerging infectious diseases and other health threats.”
“Since March 2020, the standing committee has consistently generated real-time policy recommendations and produced an unprecedented amount of timely, evidence-based guidance in response to the COVID-19 pandemic and other emerging public health threats. Looking ahead, the standing committee will continue to ensure that ASPR and decision-makers have access to the latest high-quality, evidence-based insights to inform medical and public health preparedness for, response to, and recovery from disasters and public health emergencies.”
“Approximately 12-15 volunteer experts are needed to serve on the standing committee of approximately 25 members.”
Learn more and submit nominations by December 13 here.