This week’s Pandora Report covers updates on the Trump administration’s response to the West Texas measles outbreak, challenges at NIH, possible budget cuts to DOD programs focused on WMDs and pandemic preparedness, and more.
Biodefense MS Information Session
“Prospective students are invited to attend a information session to hear more about the Biodefense M.S. program offered at the Schar School. The online session will provide an overview of the program, as well as the application process, student experience and graduate outcomes. This session admissions will be led by the Graduate Admissions team.”
This sessions will take place at 12 pm EDT on March 20. Learn more and register here.
Updates on the Trump Administration
Top HHS Officials Retire and Resign
Francis S. Collins, the well-known geneticist who ran the National Institutes of Health for 12 years, announced on Saturday that he has retired from the NIH and the federal government. Collins did not provide a reason for his departure, and he has refused to do any interviews. His parting statement offered a subtle yet pointed message to the Trump administration, with Collins writing in part “As I depart N.I.H., I want to express my gratitude and love for the men and women with whom I have worked side by side for so many years. They are individuals of extraordinary intellect and integrity, selfless and hard-working, generous and compassionate. They personify excellence in every way, and they deserve the utmost respect and support of all Americans.”
Tom Corry, Assistant Secretary of Public Affairs at HHS, also abruptly resigned from the department last Friday, just two weeks after starting his new role. Corry did not provide a reason for his departure either. He previously served as a senior advisor and Director of Communications at the Centers for Medicare & Medicaid Services during the first Trump administration.
Kennedy Embraces Unconventional Remedies as Measles Outbreak Grows
As the West Texas measles outbreak grows, HHS Secretary RFK Jr. has touted several unconventional remedies while continuing to not urge all Americans to get vaccinated against the disease. In an interview, Kennedy said the federal government is shipping doses of vitamin A to Gaines County, Texas, the epicenter of the outbreak, and helping to arrange ambulance rides. He also claimed that physicians in Texas have seen “very, very good results” treating measles with budesonide, clarithromycin, and cod liver oil. This has prompted strong backlash from many in the medical community.
As of March 6, 222 cases have been reported in Alaska, California, Florida, Georgia, Kentucky, New Jersey, New Mexico, New York City, Pennsylvania, Rhode Island, Texas, and Washington. The US eliminated measles in 2000. Today, an unvaccinated adult in New Mexico has been reported dead, a little over a week after the death of an unvaccinated child in Lubbock, Texas. The Pan American Health Organization issued an epidemiological alert in response to the outbreak earlier this week.
Bhattacharya Promises “Scientific Dissent” at NIH
Jay Bhattacharya, Donald Trump’s nominee for NIH director, said on Wednesday that NIH officials “oversaw a culture of coverup, obfuscation, and a lack of tolerance for ideas that differed from theirs” in recent years. He promised to, in response to this, “establish a culture of respect for free speech in science and and scientific dissent at the agency.”
Bhattacharya infamously co-authored the Great Barrington Declaration in October 2020, which argued for allowing people at lower risk of COVID-19 complications to go about life as normal, assuming that, if infected, they would experience mild disease and contribute to herd immunity. NPR explains that, “During the COVID pandemic, Bhattacharya clashed with the mainstream medical establishment, including the NIH, over lockdowns and other measures designed to control the spread of the virus. He says he was shunned and penalized for his views and he didn’t want anyone else to suffer the same fate.”
NIH Set to Terminate Active Research Grants
The NIH has begun mass terminations of research grants funding active scientific projects that no longer meet “agency priorities”. According to Nature News, “NIH staff members have been instructed to identify and potentially cancel grants for projects studying transgender populations, gender identity, diversity, equity and inclusion (DEI) in the scientific workforce, environmental justice and any other research that might be perceived to discriminate on the basis of race or ethnicity, according to documents and an audio recording that Nature has obtained. Grants that allot funding to universities in China and those related to climate change are also under scrutiny.”
This comes after a federal court temporarily blocked the administration’s proposed cut to NIH funding for universities’ indirect costs like facilities and administration. However, as Politico points out, the administration may pivot to renegotiating the payments with individual universities.
DOD Cuts Threaten Pandemic Preparedness, WMD Proliferation Prevention, and More
DOD agencies responsible for preventing WMD proliferation and building security capacity globally are at risk of intense budget cuts or outright abolition. According to a recent draft working paper, DOD is asking all agencies and services that oversee security cooperation programs to assess potential impacts of funding realignment. The paper was prepared in response to an RFI from Secretary of Defense Pete Hegseth that asked agencies to assess consequences of four levels of staff reduction, including 25%, 50%, 75%, and 100%. The authors of the working paper, according to WIRED, “…warn that the cuts could hobble the fight against organized crime in South America, impair the battle against the Islamic State, increase the likelihood of a rogue state producing and using chemical weapons, and defund pandemic surveillance measures.”
CDC Staff Now Prohibited from Co-Authoring Papers with WHO Personnel
Scientists at the CDC are now prohibited from co-authoring publications with WHO staff, according to reporting from HuffPost. An interim guidance document obtained by the news agency explained that “CDC staff should not be co-authors on manuscripts/abstracts with WHO staff,” while also adding that CDC staff should not author publications related to work that is “funded by WHO.” The guidance further instructs CDC staff who are lead authors on such publications to either pause all action on those publications, or to recuse themselves as authors if the publication process cannot be paused. It also says that manuscripts not in compliance with Trump’s executive orders that were submitted prior to January 20 should be withdrawn, or CDC staff should recuse themselves as authors.
US Funding Cuts Threaten Global Fight Against TB
The WHO issued a warning on Wednesday explaining that severe funding cuts (namely, those in the United States) threaten decades of progress in the global fight against tuberculosis. The agency explained that essential prevention, testing, and treatment services are collapsing, leaving millions at risk. The regions most affected include Africa, Southeast Asia, and the Western Pacific, where national TB programs depend on international support.
The TB Community Coordination Hub said in a statement about the funding cuts, “[We] strongly condemn this callous, abrupt and totally one-sided act that is unprecedented, and calls upon the US Administration to take immediate measures to restore funding and support projects globally that are crucial to contain and prevent a resurgence of this deadly disease.”
Further Reading:
- “A Call for the United States to Continue Investing in Science,” Blader et al., ASM Case Reports
- “Trump’s Foreign Aid Freeze Throws International Geneva Organisations Into Disarray,” Kasmira Jefford and Michelle Langrand, Geneva Solutions
- “CDC Firings Undermine Public Health Work Far Beyond Washington,” Rachana Pradhan, KFF Health News
- “In a Sudden Reversal, CDC Rescinds Some Staff Firings,” Pien Huang and Will Stone, NPR
- “Trump’s Data Deletions Pose a Stark Threat to Public Health,” Chrissie Giles, The Bureau of Investigative Journalism
- “Senior USAID Official Ousted as He Details Problems Providing Lifesaving Aid,” John Hudson, The Washington Post
- “The Trump Administration Said These Aid Programs Saves Lives. It Canceled Them Anyway.” Anna Maria Barry-Jester and Brett Murphy, ProPublica
- “Internal Memos: Senior USAID Leaders Warned Trump Appointees of Hundreds of Thousands of Deaths from Closing Agency,” Brett Murphy and Anna Maria Barry-Jester, ProPublica
- “KFF Poll Reveals Support for USAID, Misconceptions on Aid for Global Health,” Stephanie Soucheray, CIDRAP
- “Next World Health Assembly Should Discuss Smallpox Research Given U.S. Withdrawal from WHO,” Daniel R. Lucey, Science Speaks
- “As Measles Cases Surge in Texas, WHO’s Global Control Program Risks Collapse,” Jason Gale, MSN
- “On COVID’s Fifth Anniversary, Scientists Reflect on Mistakes and Successes,” Tanya Lewis, Scientific American
CDC Monitoring Mysterious Disease in DRC
In a statement on Tuesday, the CDC said it is closely monitoring the outbreak of an unknown disease that has already killed dozens in the Democratic Republic of Congo. According to WHO, at least 1,318 people have exhibited symptoms of the disease, and 60 had died from it by February 27. A new mpox variant was also recently discovered in the country. The new variant has a mutation known as APOBEC3, which indicates it may be more easily transmissible than previously identified strains.

“WHO Technical Advisory Group on the Responsible Use of the Life Sciences and Dual-Use Research: Report of the Meeting, 30 October 2024”
From WHO: “The World Health Organization (WHO) Technical Advisory Group on the Responsible Use of the Life Sciences and Dual-Use Research (TAG-RULS DUR) was established to provide independent advice to WHO on the monitoring and mitigation of biorisks, the advances in the life sciences and related technologies, the governance of dual-use research and the responsible use of the life sciences. This report summarizes the meeting that was virtually held on 30 October 2024. Over the course of the meeting, participants discussed and provided feedback on activities to operationalize the framework and delivered updates on activities of the TAG-RULS DUR’s four working groups.”
“A WHO Global Framework to Guide Investigations Into Origins of Potentially Epidemic and Pandemic Pathogens”
The Scientific Advisory Committee for the Origins of Novel Pathogens (SAGO) and WHO SAGO Secretariat recently published this comment in Nature, writing in its introduction “In outbreak situations involving a novel pathogen timely and coordinated response is crucial. The WHO Scientific Advisory Group for the Origins of Novel Pathogens recently released a global framework to guide future scientific investigations into the origin of emerging pathogens.”
“Recent Virus Research Should Raise the Alarm”
W. Ian Lipkin and Ralph Baric recently published this opinion piece in The New York Times: “There’s a central question that many scientists face: How can scientific discoveries drive humanity’s progress without posing a dire risk to it? As virus experts, we’re committed to research that uncovers pandemic threats and helps protect people from them. But we are concerned about how some scientists are experimenting with viruses in ways that could put all of us in harm’s way.”
“From Inception to Fielding: Meeting the Challenges of Medical Countermeasure Development”
Sarah M. Wiles recently published this article in CBNW: “The U.S. Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear Defense has a robust development process for new CBRN medical countermeasures. Sarah M. Wiles explains the process and its rationale.”
“Automated Grading for Efficiently Evaluating the Dual-Use Biological Capabilities of Large Language Models”
Bria Persaud, Ying-Chiang Jeffrey Lee, Jordan Despanie, Helin Hernandez, Henry Alexander Bradley, Sarah L. Gebauer, Greg McKelvey, Jr. recently published this RAND Corporation working paper: “The authors of this working paper developed a proof-of-concept automated grader and used it to assess large language models’ abilities to answer knowledge-based questions and generate protocols that explain how to perform common laboratory techniques that could be used in the creation of proxies for biological threats.”
“UNIDIR Empowers Emerging Leaders in Biological Disarmament and Biosecurity”
From UNIDIR: “As the world marks the 50th anniversary of the Biological Weapons Convention (BWC), UNIDIR alongside the DiploFoundation and the Fondation pour la Recherche Stratégique celebrates the successful completion of the inaugural BWC Advanced Education Course (BWCedu). This five-month advanced training programme – funded by the UK’s Foreign, Commonwealth & Development Office – brought together 25 emerging leaders from a diverse range of States, with a focus on participants from the Global South.”
Read here.
“Seven Years Since Salisbury Was Centre of Novichok Attack”
Isabella Holliday recently authored this news article about the anniversary of the Novichok attack targeting the Skripals in Salisbury, UK. Read it in the Salisbury Journal here.
“Syria’s Caretaker Foreign Minister Addresses OPCW’s Executive Council”
From OPCW: “In a landmark visit to OPCW’s Headquarters in The Hague, caretaker Foreign Minister al-Shaibani reaffirmed the commitment of the new Syrian authorities to cooperate with the OPCW to eliminate the chemical weapons programme of the former Syrian regime”.
Read here.

ICYMI: The Cost of Defunding PEPFAR and the Impact on the Fight Against HIV/AIDS
Brown’s Pandemic Center hosted this webinar in late February. Watch the recording here. Key topics included:
“Call for Action – How policymakers, philanthropists, and institutions can mobilize to address these urgent gaps.”
“PEPFAR Changes & Uncertainty – Concerns about funding gaps, particularly affecting pediatric HIV treatment, maternal health, and job losses in healthcare.”
“Impact on South Africa & Beyond – The success of PEPFAR in South Africa and the potential consequences of its reduction, including rising HIV cases and strain on health systems.”
“Future of Global Health Funding – Exploring alternative funding sources, the role of UNICEF, private sector involvement, and the need for governments to step up.”
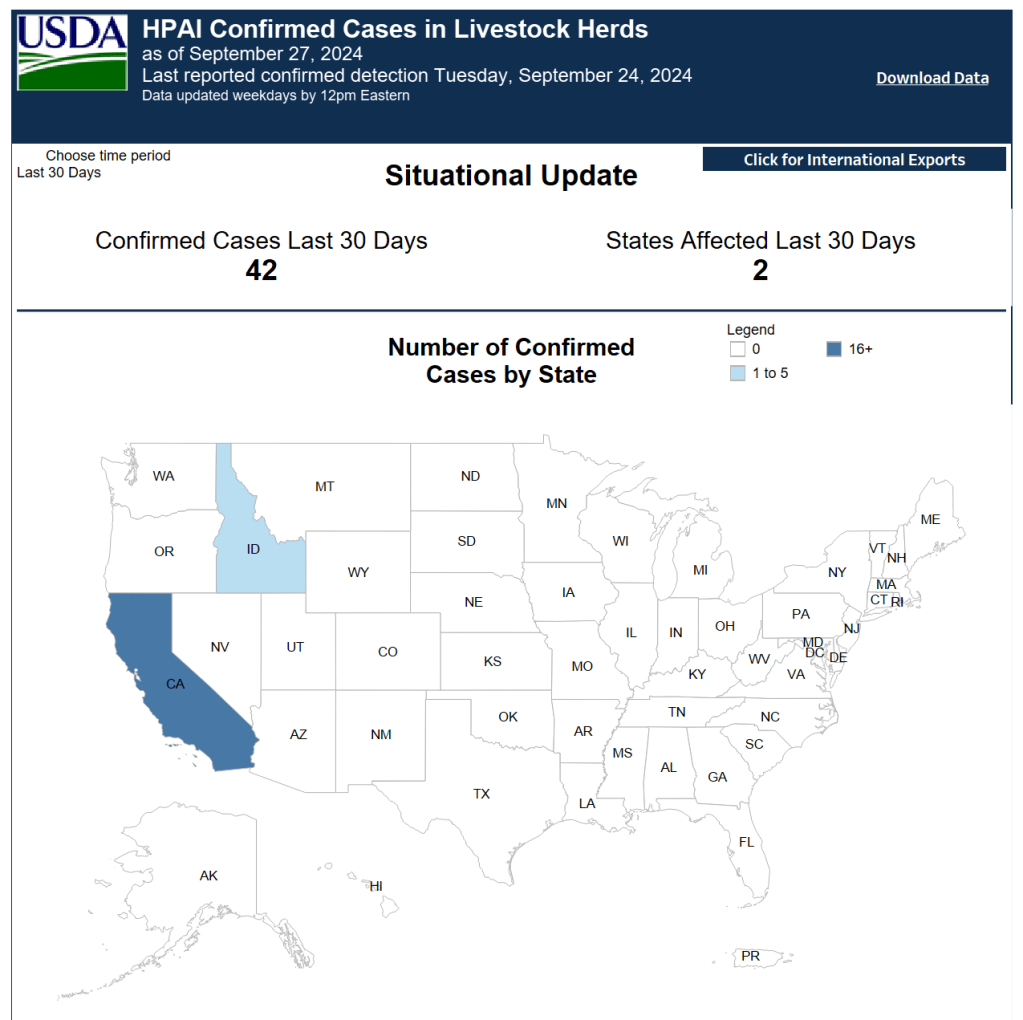
NEW: Building Trust in the H5N1 Response: Perspectives from the Field
From NASEM: “Since avian influenza (H5N1) was first detected in dairy cattle in March 2024, H5N1 has resulted in human infections, diminished livestock production, and decimated wildlife populations. Uncoordinated policies at the national, state, and local levels have challenged mitigation efforts, and mistrust has hindered the urgent response needed for the rapidly evolving threat. The National Academies Forum on Microbial Threats will host a public webinar on March 27 where agricultural producers and workforce health specialists will explore strategies to build greater mutual trust and a coordinated One Health response.”
This webinar will take place on March 27 at 2 pm ET. Register here.
2025 Scowcroft Institute Pandemic Policy Summit
From the Scowcroft Institute: “The Scowcroft Institute of International Affairs at Texas A&M University invites you to attend the 2025 Scowcroft Institute Pandemic Policy Summit examining the ongoing H5N1 outbreak across the U.S. Dairy industry. This summit will bring together experts from government, academia, and industry to review the response efforts, discuss current challenges and opportunities, and identify options for moving forward. Listen to panels of subject matter experts, explore case studies from the field, and participate in networking opportunities.”
This event will take place on March 18 in Washington, DC. Learn more and RSVP here.
Sustainable Manufacturing: Building and Preserving a Resilient Medical Industrial Base
“Join industry and government partners for our second annual industry summit! During this event, leaders from IBMSC will share our strategic vision and organizational priorities. Speakers will also share potential opportunities for building and preserving the medical industrial base. This event will be in-person only and space is limited!”
This event will take place March 11-12 in Washington, DC. Learn more and register here.
Gaming Weapons of Mass Destruction Course – From Policy to Practice
From MORS: “Gaming Weapons of Mass Destruction (WMD – defined as Nuclear, Chemical, and Biological agents) will be a three-day course focused on developing and executing games related to WMD in all its forms. While the basics of WMD capabilities and game design will be discussed, this will be a course focused on the intersection of WMD and gaming. It will not be either a WMD or gaming course; for those topics see other offerings.”
“No prior experience is required for this course, though a basic familiarity with various agents and their effects would be helpful, as would a basic understanding of professional gaming and how it is used. The instructors will adapt in real time to class requirements (e.g., if the class is interested in animal and plant targets, the instructors have extensive experience in designing games on those subjects as well).”
This course will take place March 18-20 on Zoom. Learn more and register here.

NEW: Apply for the 2025 Youth for Biosecurity Fellowship
“The global norm against biological weapons cannot be maintained without the inclusion of youth voices in the multilateral discussions taking place in the framework of the Biological Weapons Convention (BWC). Youth perspectives are key to create innovative solutions and generate long-term engagement. There are benefits to including the perspectives of young people from developing countries, where over 90% of the world’s youth reside.”
“Organized by the United Nations Office for Disarmament Affairs in Geneva, in partnership with key international actors that empower youth in science diplomacy and global biosecurity, the Youth for Biosecurity Fellowship provides a unique learning and networking experience in the framework of the Biological Weapons Convention.”
“Launched in 2019 as a Biosecurity Diplomacy Workshop, the Youth for Biosecurity Initiative organized its first fellowship in 2023. For the third edition, the fellowship will provide the opportunity for 20 competitively selected young scientists from the Global South to join an online interactive training programme prior to a field visit during the meeting of the BWC Working Group on the Strengthening of the Convention in Geneva.”
Learn more and apply by April 7 here.
NASEM Has Questions
The National Academy of Science, Engineering, and Medicine (NASEM) is hosting a workshop on Navigating the Benefits and Risks of Publishing Studies of In Silico Modeling and Computational Approaches of Biological Agents and Organisms on April 3-4 in Washington, DC. In preparation for the workshop, NASEM is soliciting input on how publishing computational models can support biological research while minimizing potential DURC/PEPP risks. The purpose of the questionnaire is to ascertain if organizations that publish or disseminate scientific knowledge have considered or created guidelines or policies to review, host or interact with in silico and computational models and tools, studies, datasets, etc. research that constitutes dual use, dual use research of concern (DURC) or pathogens with enhanced pandemic potential (PEPP). Have answers? Then fill out the In Silico Research Publications Pre-event Questionnaire.
NOFO, Addressing Agricultural Biorisk Evidence Base Gaps with Applied Research
“There is a global recognition that the current evidence base to inform laboratory biological risk management has gaps, and that biosafety and biosecurity policies are not always based on evidence.1 This notice of funding will support the design and implementation of applied biorisk research to address evidence gaps in working with high-consequence veterinary and agricultural pathogens as identified during the RAV3N Biorisk and Biosafety Gap Assessment Workshop2 or similar gap analysis like the WOAH working group agent specific biorisk gap analysis.1 ERGP is seeking proposals that address one or more key focus area components listed below. Each proposal will go through an internal ERGP and external expert review. Successful proposals should address at least one of the three key focus areas and at least one component under that area.”
“This funding opportunity aims at the design and implementation of applied biorisk research to address evidence gaps in working with high-consequence veterinary and agricultural pathogens.”
“This work will contribute to recommended guidance on laboratory biosafety and agricultural biosecurity, using research techniques to evaluate the application and effectiveness in operational contexts. All proposals must make a clear experimental plan for how the applicant will test the application and outcomes of their focus area(s)/component(s) in their facility.”
Learn more and submit application by April 14 here.